The commercial preparation of sodium hydroxide (NaOH) is primarily done through the chloralkali process. This process involves the electrolysis of sodium chloride (NaCl) solution (brine) to produce sodium hydroxide, chlorine gas, and hydrogen gas.
Chemistry of the Process:
-
Electrolysis of Brine: Brine is subjected to electrolysis in an electrolytic cell with a diaphragm or membrane separating the anode and cathode compartments.
-
At the Anode (Oxidation): Chloride ions (Cl⁻) are oxidized to chlorine gas (Cl₂):
2Cl−→Cl2(g)+2e−2Cl^- \rightarrow Cl_2 (g) + 2e^-
-
At the Cathode (Reduction): Water is reduced to produce hydrogen gas (H₂) and hydroxide ions (OH⁻):
2H2O+2e−→H2(g)+2OH−2H_2O + 2e^- \rightarrow H_2 (g) + 2OH^-
-
-
Overall Reaction:
2NaCl+2H2O→2NaOH+Cl2+H22NaCl + 2H_2O \rightarrow 2NaOH + Cl_2 + H_2
Sodium hydroxide (NaOH) is formed in the electrolyte solution.
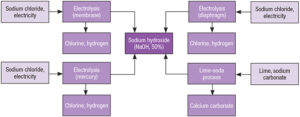
Products:
-
Sodium Hydroxide (NaOH): The sodium hydroxide is collected in the cathode compartment.
-
Chlorine Gas (Cl₂): Produced at the anode.
-
Hydrogen Gas (H₂): Produced at the cathode.
Applications:
-
Sodium hydroxide is used in the manufacture of soap, paper, textiles, and various chemicals.
-
Chlorine is used in disinfectants, plastics, and chemical synthesis.
-
Hydrogen is used as a fuel and in the production of ammonia for fertilizers.