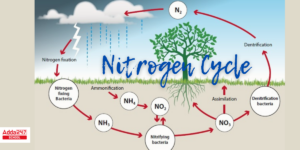
The Nitrogen Cycle
The nitrogen cycle is a crucial biogeochemical cycle that describes the movement of nitrogen through the atmosphere, lithosphere, hydrosphere, and biosphere. Nitrogen is essential for life, particularly for the formation of proteins, nucleic acids, and other cellular components. Despite making up 78% of Earth’s atmosphere, nitrogen in its molecular form (N₂) is not directly usable by most organisms, and it must undergo various transformations in the nitrogen cycle before it can be utilized.
Steps in the Nitrogen Cycle
-
Nitrogen Fixation:
- Nitrogen fixation is the process by which nitrogen (N₂) from the atmosphere is converted into ammonia (NH₃) or nitrates (NO₃⁻), forms that can be used by plants.
- Biological Nitrogen Fixation: Certain bacteria, such as Rhizobium (found in the root nodules of legumes) and Azotobacter (in soil), convert nitrogen gas into ammonia. These bacteria have the enzyme nitrogenase, which facilitates the conversion of N₂ into usable nitrogen compounds.
- Abiotic Nitrogen Fixation: Lightning and industrial processes (e.g., the Haber-Bosch process) can also convert atmospheric nitrogen into nitrates, which then enter the soil.
-
Nitrification:
- Nitrification is a two-step process in which ammonia is first converted into nitrites (NO₂⁻) and then into nitrates (NO₃⁻).
- Step 1: Ammonia (NH₃) is oxidized to nitrites (NO₂⁻) by bacteria such as Nitrosomonas.
- Step 2: Nitrites (NO₂⁻) are further oxidized into nitrates (NO₃⁻) by bacteria like Nitrobacter. Nitrates are easily absorbed by plants to make proteins and other nitrogen-containing compounds.
-
Assimilation:
- In the assimilation process, plants absorb nitrates (NO₃⁻) or ammonia (NH₃) from the soil and use them to form amino acids, proteins, and other nitrogenous compounds. Herbivores, in turn, consume the plants, incorporating nitrogen into their own bodies.
-
Ammonification (Decomposition):
- When plants, animals, and other organisms die, decomposers such as bacteria and fungi break down their organic matter. During this process, organic nitrogen compounds (e.g., proteins and nucleic acids) are converted into ammonia (NH₃) or ammonium ions (NH₄⁺), a process known as ammonification.
- The ammonia produced can be further used by plants or transformed into nitrites and nitrates by nitrifying bacteria.
-
Denitrification:
- Denitrification is the process in which nitrates (NO₃⁻) are converted back into nitrogen gas (N₂) by denitrifying bacteria, such as Pseudomonas and Clostridium, under anaerobic conditions (when oxygen is absent). This process completes the nitrogen cycle by returning nitrogen to the atmosphere, maintaining the balance of nitrogen in the ecosystem.
Key Points in the Nitrogen Cycle
- Nitrogen Fixation: Converts atmospheric N₂ into ammonia (NH₃) or nitrates (NO₃⁻) that plants can use.
- Nitrification: The conversion of ammonia into nitrites and then nitrates, making nitrogen available to plants.
- Assimilation: Plants and animals incorporate nitrogen into their bodies for growth and reproduction.
- Ammonification: Decomposers break down dead organisms, releasing nitrogen back into the soil as ammonia.
- Denitrification: Bacteria convert nitrates back into nitrogen gas, returning nitrogen to the atmosphere.
Human Impact on the Nitrogen Cycle
Human activities, such as agriculture, industry, and the burning of fossil fuels, can disrupt the nitrogen cycle in several ways:
-
Excessive Fertilizer Use: The use of synthetic fertilizers adds large amounts of nitrogen to the soil in the form of nitrates, which can leach into waterways, causing eutrophication and algal blooms in aquatic ecosystems.
-
Burning of Fossil Fuels: The combustion of fossil fuels releases nitrogen oxides (NOₓ) into the atmosphere, which can contribute to air pollution, acid rain, and smog.
-
Deforestation: The removal of forests reduces the amount of nitrogen fixation that occurs naturally through plant-bacteria symbiosis and disrupts the overall nitrogen balance in ecosystems.
-
Livestock Farming: Livestock farming produces large amounts of nitrogen in the form of ammonia (from animal waste) and methane (a potent greenhouse gas), which can affect soil and air quality.