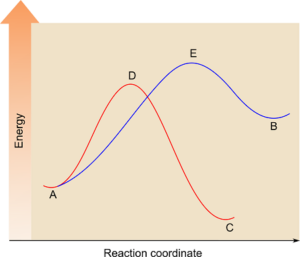
In an exothermic reaction, the reaction profile typically shows a decrease in energy from reactants to products. The faster reaction will have a lower activation energy and will reach the product state quicker.
FreeNotes
In an exothermic reaction, the reaction profile typically shows a decrease in energy from reactants to products. The faster reaction will have a lower activation energy and will reach the product state quicker.
