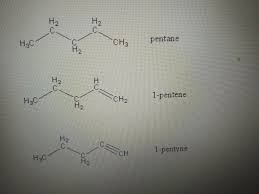
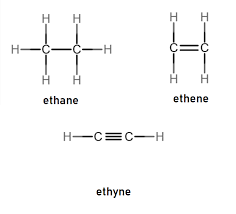
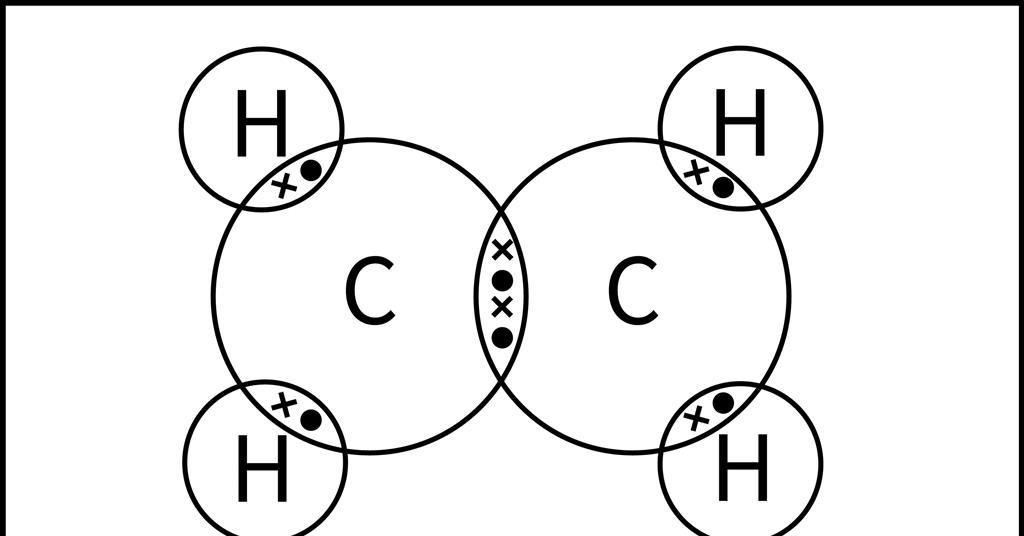
What is the effect of adding bromine water to an alkene?
When bromine water is added to an alkene, the brown color of the bromine water disappears, indicating that the alkene undergoes addition reaction with bromine (forming a dihaloalkane). Related Questions: Give three examples of unsaturated hydrocarbons. Draw electron dot and cross structure for ethene. How can you differentiate ethane from ethene? What do you mean … Read more